Post-Translational Modifications
Almost all proteins undergo post-translational modifications. The term post-translational modification (PTM) refers to modifications that occur during or after translation of the polypeptide chain.
Symansis' proteins are expressed in modified human 293 cells. Since the cells are human, they have the cellular machinery necessary to attach human post-translational modifications to the proteins being expressed.
PTMs include:
- covalent modifications, e.g. phosphorylation, methylation and glycosylation
- proteolytic processing, e.g. removal of signal and/or propeptides
- non-enzymic modifications, e.g. deamidation and racemisation
The type and position of the PTMs are generally considered to be important. It is known that they can play a role in protein folding, biological activity, cell targeting and immunogenicity.
There are some specific examples where the presence of certain PTMs has been found to be critical. For example, Rajarathnam et al. (1998) discovered that alterations to the 7-34 disulfide bridge in IL-8 resulted in "a dramatic reduction in biological potency". Similarly, de Jonge et al. (2000) showed that N-myristoylation of tBID and SOS3 is essential for the proper functioning of these proteins.
One of the best-studied examples of the necessity of PTMs is the glycosylation of erythropoietin. In 1988, Dube et al. determined that glycosylation at Asn-38, Asn-83 and Ser-126 is essential for proper biosynthesis and secretion of the mature protein. Since this discovery many researchers have investigated the role of EPO glycosylation in vivo and in vitro [e.g., Wasley et al. (1991), Elliott et al. (2004)].
Recombinant proteins are dependent on the machinery of the cell line to determine what PTMs are attached. For example, E. coli does not possess the same type of cellular machinery used for glycosylation in higher organisms like humans. Therefore, human proteins expressed in E. coli are not glycosylated
Post-translational modifications result in protein heterogeneity. The protein exists in multiple isoforms, which differ according to their level of post-translational modification. Expression of these isoforms is highly significant for cell biology, as they more closely resemble the native human proteins.
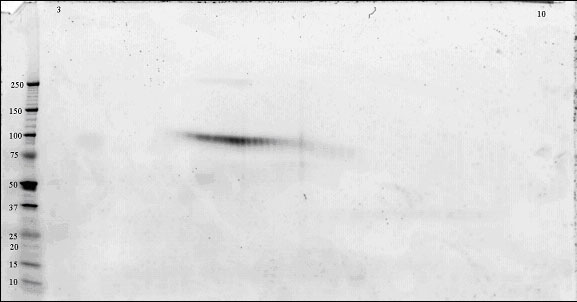
The preferred way to visualise these protein isoforms is by 2-dimensional electrophoresis (2-DE). 2-DE gels separate proteins by charge in the first dimension and by molecular weight in the second dimension. Protein isoforms typically appear as "spot trains" [Gooley et al. (1997)]. This is particularly true for glycoproteins that have isoforms that vary in pI (due to addition of sialic acid) and molecular weight (due to addition of different size and number of glycan chains).
An example of a 2-DE gel image is shown below.