Human Cell Expression Results in Human Glycosylation
Recombinant proteins are dependent on the glycosylation machinery of the cell line to determine what glycan structures are attached. As E. coli does not possess the same type of cellular machinery used for glycosylation in higher organisms like humans, human proteins expressed in E. coli are not glycosylated.
Human proteins expressed in rodent cell lines e.g. CHO and NSO, and the insect cell line Sf21 exhibit glycosylation structures that are distinct from those found on the native protein. For example human erythropoietin (EPO) expressed in BHK-21 and CHO cells have elongated oligosaccharide branches that are not found on native EPO isolated from human plasma.
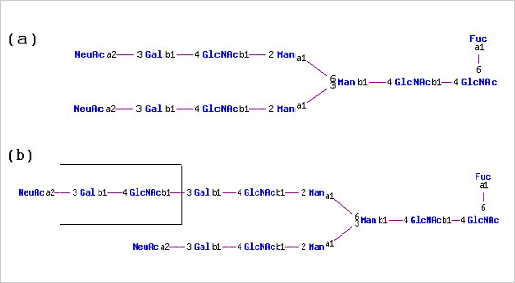
(a) N-linked glycan structure typically found on native human EPO (b) N-linked glycan structure found on recombinant human EPO expressed in CHO cells. Note that the Gal(b1-4)GlcNAc(b1-3) repeat found in CHO expressed EPO is not found in N-glycan structures on native human EPO.
Similarly, tissue plasminogen activator isolated from human Bowes melanoma cells has N-linked glycans with predominately GalNAc(b1-4)GlcNAc(b1-2/6)Man branches. The same protein made recombinantly in mouse C127 cells had N-linked glycans with mainly Gal(b1-4)GlcNAc(b1-2)Man branches and high-mannose structures, not found on the native human protein.
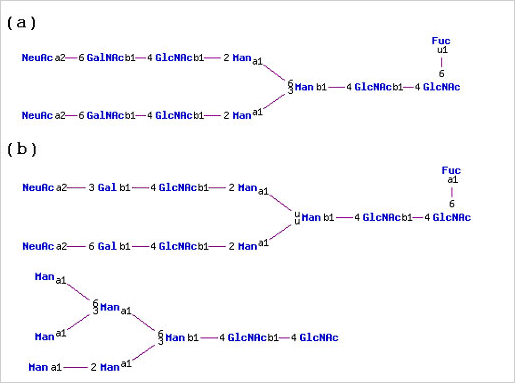
(a) N-linked glycan structure found on human tissue plasminogen activator isolated from human Bowes melanoma cells (b) N-linked glycan structures found on recombinant human tissue plasminogen activator expressed in mouse C127 cells.
NB. Structures found on proteins taken from GlycoSuiteDB (www.glycosuite.com)
