Symansis' Human Interferons
Human-specific glycosylation of human interferons
Interferons (IFNs), initially discovered fifty years ago as virus inhibiting agents1, are now known to be a family of pleiotropic cytokines with additional antiproliferative, antitumour, and immunomodulatory properties. Current clinical uses of IFNs include treatment of viral infections, cancers and autoimmune diseases2. The clinically relevant IFNs include IFN-alpha 2a and 2b, IFN-beta and IFN-gamma. IFN-alpha 2 isoforms are used to treat malignant melanoma, hairy cell leukemia, chronic hepatitis B, chronic hepatitis C, follicular (non-Hodgkin's) lymphoma, and AIDS-related Kaposi's sarcoma. IFN-beta has been approved for treatment of multiple sclerosis whereas IFN-gamma is used to treat chronic granulomatous disease and osteopetrosis.
At present, all commercial sources of therapeutic IFNs are produced from E.coli, except IFN-beta 1a, which is produced from CHO cells3. IFNs occur naturally as glycoproteins, although the effect of glycosylation on the in vivo activity of IFNs has not been extensively characterised. Nevertheless, it is known that glycosylation has major effects on pharmacokinetics, solubility, stability, and receptor binding of therapeutic proteins.4
In particular, glycosylation of IFN-beta has been associated with 10-fold higher antiviral, antiproliferative and immunomodulatory activity. Reduced antiviral activity has been attributed to the tendency of IFN-beta 1b to form large, insoluble aggregates due to the lack of glycosylation5 as the glycans have a stabilising effect on the structure. Glycosylation is also critical for human IFN-gamma protease resistance6. Thus glycosylation is of critical importance for IFN function and therefore using human cell expressed proteins could potentially be beneficial for experimental outcomes.
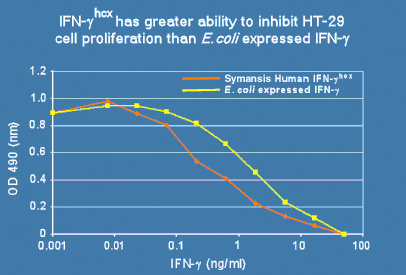
Symansis' recombinant hcx™ proteins are unique in being produced from human cells and containing human-specific post-translational modifications including glycosylation, making them more similar to naturally occurring proteins compared to E.coli or CHO expressed recombinant human proteins. Symansis produces human cell expressed (hcx™) IFN-alpha 2b and IFN-gamma as well as the IFN-gamma receptor 1 extracellular domain fused to the Fc region of human IgG1. IFN-gammahcx, for example, has greater antiproliferative activity than E. coli expressed IFN-gamma (Figure 1), demonstrating the advantage of using human cell expressed IFNs.
References
1. Isaacs and Lindermann (1957) Proc. R. Soc. Lond. B Biol.Sci. 147:258-267
2. Maher et. al. (2007) Curr Med Chem. 14:1279-1289
3. Walsh G. (2006) Nature Biotechnology 24:769 – 776
4. Werner et. al. (2007) Acta Paediatr Suppl. 96:17-22
5. Runkel et. al. (1998) Pharm Res 15:641-649
6. Sareneva et. al. (1995) 308:9-14
