Vargatef
Catalog Number: SY-Vargatef
Availability: In Stock
Size:
25 mg, 100 mg
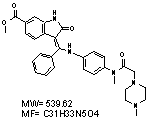
CAS Number : 656247-17-5
Alternative Names: Vargatef, BIBF-1120, BIBF1120, Nintedanib,Intedanib,928326-83-4, CHEMBL502835,Ofev
Availability: In Stock
Alternative Names: Vargatef, BIBF-1120, BIBF1120, Nintedanib,Intedanib,928326-83-4, CHEMBL502835,Ofev
Qty:
| Intedanib is an indolinone-derived drug that inhibits the process of blood vessel formation (angiogenesis) in tumors. Preclinical studies have demonstrated that intedanib selectively binds to and blocks the VEGF, FGF and PDGF receptors, inhibiting the growth of cells that constitute the walls of blood vessels (endothelial and smooth muscle cells and pericytes) in vitro. Intedanib reduces the number and density of blood vessels in tumours in vivo, resulting in tumour shrinkage. Intedanib also inhibits the growth of cells that are resistant to existing chemotherapy agents in vitro, which suggests a potential role for the agent in patients with solid tumours that are unresponsive to or relapse following current first line therapy. Early clinical trials of intedanib have been carried out in patients with non-small cell lung, colorectal, uterine, endometrial, ovarian and cervical cancer and multiple myeloma. These studies reported that the drug is active in patients, safe to administer and is stable in the bloodstream. They identified that the maximum tolerated dose of intedanib is 20 0 mg when taken once a day. In the first human trials, intedanib halted the growth of tumours in up to 50% of patients with non-small cell lung cancer and 76% of patients with advanced colorectal cancer and other solid tumours. A complete response was observed in 1/26 patients with non-small cell lung and 1/7 patients with ovarian cancer treated with Intedanib. A further 2 patients with ovarian cancer had partial responses to intedanib. Two phase II trials have been carried out assessing the efficacy, dosing and side effects of Intedanib in non-small cell lung and ovarian cancer. These trials found that intedanib delayed relapse in patients with ovarian cancer by two months, and that overall survival of patients with non-small cell lung who received Intedanib was similar to that observed with the FDA approved VEGFR inhibitor sorafenib. These trials also concluded that increasing the dose of the intedanib has no effect on survival. Intedanib is currently undergoing investigation in phase II and III clinical trials and is yet to be licensed by the FDA. Angiogenesis inhibitors such as intedanib may be effective in a range of solid tumour types including; lung, ovarian, metastatic bowel, liver and brain cancer. Several further phase I and II clinical trials with intedanib are underway. Patients are also being recruited for three phase III clinical trials that will evaluate the potential benefit of intedanib when added to existing 1st line treatments in patients with ovarian, and 2nd line treatment in non-small cell lung cancer. The phase III trials of intedanib in lung cancer have been named LUME-Lung 1 and LUME-Lung 2. See Here for more details regarding this drug and its use. |
 |
