HGF
Availability: In Stock
Species Reactivity : Human
Host : Human, HEK Cell Expessed
|
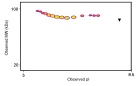
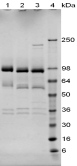
Hepatocyte growth factor (HGF) consists of two subunits held by a disulfide bond. The alpha subunit of HGF (MW 69 kDa) has a length of 440 amino acids and the beta subunit (MW 34 kDa) has a length of 234 amino acids. Both subunits of HGF are obtained by endoproteolytic cleavage of a common preproprotein of 728 amino acids (approximately 90 kDa) which itself is biologically inactive. Maturation of the precursor into the active heterodimer takes place in the extracellular environment and results from proteolytic cleavage by urokinase which acts as a HGF convertase. Densitometry of protein isoforms visualised by 2-DE. Spot trains are normal in human proteins and highlight the presence of multiple isoforms which differ according to their level of post-translational modification. The triangle indicates the theoretical MW and pI of the protein. HGF is produced predominantly in Kupffer cells and sinusoidal endothelial cells of the liver and in the pancreas. HGF synthesis is also stimulated in particular by injuries affecting liver tissues (hepatitis, ischemia, hepatectomy) and can be isolated from platelets, kidney, human serum and placenta. HGF stimulates the proliferation of hepatocytes in vivo and is the most potent known mitogen for hepatocytes in primary culture. HGF is also a mitogen for kidney epithelial cells. HGF has been shown also to be one of the priming factors for neutrophils. HGF can also lead to the formation of tubular structures in growing epithelial cells. HGF is known to synergize with IL3 and GM-CSF to stimulate colony formation of hematopoietic progenitor cells in vitro and thus may modulate hematopoiesis. HGF has also been shown to have neurotrophic activity. 1D SDS-PAGE of Symansis' hcx protein before and after treatment with glycosidases to remove oligosaccharides. A drop in the observed MW after treatment indicates the presence of glycosylation. 1. Jiang et al. (2005) Crit Rev Oncol Hematol. 53(1):35-69. |
 |
 |
